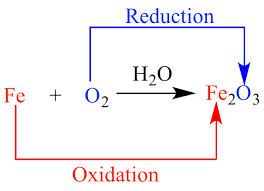
Rust forms when oxygen reacts with iron but simply putting iron and oxygen together isnt sufficient. This would result in creating iodide with the help of a starch indicator to.
Redox Reactions Meaning Characteristics And Examples
Lets discuss these two components separately then circle back to how they combine in a full redox reaction.
. Redox Reaction in Combustion. The reaction progresses forward and backward at the same rate meaning there is no net electron flow. The cell potential and free energy example shows how to calculate free energy of a redox reaction.
It is also shorthand for oxidation reduction reaction. An explosion is a fast form of combustion. An oxidation-reduction redox reaction is a type of chemical reaction that involves a transfer of electrons between two species.
Although about 21 of air consists of oxygen rusting doesnt occur in dry air. The entire reaction can be split into two half-reactions and in the case of an electrochemical cell one half-reaction occurs at the anode the other at the cathode. The combination of ammonium perchlorate and powdered aluminium inside the rocket.
Zn Zn 2 2e. Behaviour a feeling or an action that is a direct result of something else. A reaction between hydrogen gas and fluorine gas is the example of a redox reaction.
It also involves the use of a potentiometer or a redox indicator. With no electron flow. Redox is a shorthand for reduction-oxidation meaning that a redox reaction is one in which both a reduction reaction and an oxidation reaction takes place at once.
H 2 g F 2 g 2HFg In the above-mentioned reaction the hydrogen is oxidized from 0 to 1 oxidation state and so this is the reducing agent. Although rust is considered the result of an oxidation reaction its worth noting not all iron oxides are rust. Redox potential is expressed in volts V.
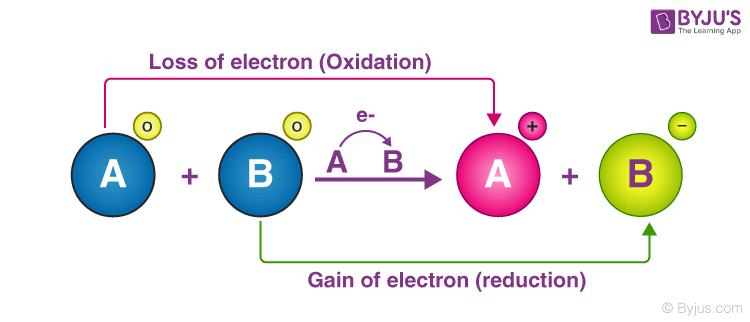
An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule atom or ion changes by gaining or losing an electron. The technical chemical term for a reaction that involves the exchange of electrons is a reduction-oxidation reaction more commonly called a redox reaction. As it is reduced from 0 to -1 fluorine is the oxidizing agent.
Types of Electrochemical Cell. In electrochemistry spontaneous reaction redox reaction results in the conversion of chemical energy into electrical energy. Flow batteries store the electrolytes in external tanks away from the battery center VRFBs generally use two such.
However the irreversible oxygen release causes. The oxygen redox reaction in lithium-rich layered oxide battery cathode materials generates extra capacity at high cell voltages ie 45 V. Redox potential also known as oxidation reduction potential ORP pe or is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respectively.
Oxidation is the loss of electrons or an increase in the oxidation state of a chemical or atoms within it. An example of this type of titration is treating an iodine solution with a reducing agent. 1 that a RFB generally includes two tanks containing the catholyte and anolyte with redox species a reaction chamber with two electrodes where redox reactions of the redox species take place an ion selective membrane and pumps for the fluids flow.
These interconversions are carried out in equipment called electrochemical cell. The half reaction for the oxidation reaction omitting phase labels is as follows. In the above reaction the oxidation reaction is shown by hydrogen as its oxidation number changes from 0 to 1 on the other hand reduction reaction is shown by fluorine as its oxidation number changes from 1 to 0.
It is a redox reaction because the Oxidation number shows an increase and decrease of the reactants. The Chemical Reaction That Forms Rust. Even the space shuttle uses redox reactions.
The reaction between magnesium oxide and carbon at 2000C to form magnesium metal and carbon monoxide is an example of the reduction of magnesium oxide to magnesium metal. Redox reductionoxidation ˈ r ɛ d ɒ k s RED-oks ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of substrate change. Hence explosion can be treated as a redox reaction.
As it is evident from Fig. Redox titration is a type of titration which is based on a redox reaction between the analyte and the titrant. Reduction is the gain of electrons or a decrease in the oxidation state of a chemical or atoms within it.
This half reaction is balanced in terms of the number of zinc atoms and it also shows the two electrons that are needed as products to account for the zinc atom losing two negative charges to become a 2 ion. Reduction is the gain of electrons and is what. Combustion is a type of oxidation-reduction reaction and hence it is a redox reaction.
If ΔG 0 solve for E cell 0 -nFE cell E cell 0 V This means at equilibrium the potential of the cell is zero. For example the more positive the reduction. Redox reactions are common and vital to some of the basic functions of life including photosynthesis.
The term reduction comes from the Latin stem meaning to lead back Anything that that leads back to magnesium metal therefore involves reduction. Each species has its own intrinsic redox potential. With half reactions there is.
The most promising commonly researched and pursued RFB technology is the vanadium redox flow battery VRFB One main difference between redox flow batteries and more typical electrochemical batteries is the method of electrolyte storage. In principle the concentration and redox potentials of catholyte and anolyte directly. The reverse process is also possible where a non-spontaneous chemical reaction occurs by supplying electricity.

Spm Chemistry Redox Reaction Define Oxidation And Reduction Youtube
Redox Reactions Examples Types Applications Balancing

Redox As Reduction And Oxidation Chemistry Class 11 Iit Jee Main Advanced Aipmt Askiitians Youtube

0 Comments